What Is the Mole Ratio of Co2 to H2o
1 mol O_2 2 mol H_2 1 mol O_2 or 1 mol O_2 2 mol H_2 1 mol O_2. 31 NH3CO2 gives excess of ammonia.

Solved 7 Balance The Equation For The Synthesis Of Sucrose Chegg Com
What is the mole ratio between all compounds of this reactionHC2H3O2 NaHCO3 NaC2H3O2 H2O CO2.
. Chemistry questions and answers. 2 mol H_2O The mole ratio for O_2 H_2O is. The chemical equation is as follows.
2 C4H10 13 O2 8 CO2 10 H2O. 1 mol O_2. How many grams of H2O are produced if 689 g of O2 are combined with excess carbon dioxide.
The molar ratio of O2 CO2 is 138 O2H2O is 1310 C4H10 CO2 is 14 and C4H10 H2O is 15. How many grams of CH4 are produced if 22 mol of CO2 are combined with excess oxygen. The mole concept is the concept of how many moles of a reactant reacts with how many moles of the reagent to produce how many moles of productIt gives us the stoichiometric ratio of the reactants with the products.
2 C4H10 13 O2 8 CO2 10 H2O. Given the reactant amounts specified in each chemical eguation determine the limiting reactant in each case. So CO2 is limiting reactant.
2 C4H10 13 O2 8 CO2 10 H2O. 2 mol H_2O 1 mol O_2. See answer 1 Best Answer.
What is the mole ratio of O2 to CH4. What is the mole ratio of O2 to moles of H2O. 500 mol H₂O 1molO₂ 2molH ₂O 250 mol O₂.
Jan 15 2014. 21 forms 11 ratio for above 3. If the question had been stated in terms of grams you would have had to convert grams of H₂O to moles of H₂O then moles of H₂O to moles of O₂ as above and finally moles of O₂ to grams of O₂.
What is the mole ratio of O2 to CH4. 2 mol H_2. Note that the ratio of moles of CO2 produced to moles ofNaHCO3 reacted is 12.
Answer11ExplanationFrom the equation we see that there is one mole of methane and one mole of carbon dioxide. CH4 O2 -. This problem has been solved.
So in one mole of carbon dioxide there will be two moles of oxygen. Answer 1 of 2. So total number of moles of Oxygen will.
How many grams of CO2 are produced if 12 g of O2 is combined with excess methane. HCLNaOH-NaClH2O 20 mole of HCl 25 mole NaOH b. 6 mol-2418 kJmol-14508 kJ.
Thus X is C 5 H 8 C n H 2 n 2. 477g of urea6006 gmole 079mole of urea 4. Means the compound has 5 carbons and 8 hydrogens.
CH4 O2 -. In the reaction shown what is the mole ratio that would be used to determine the number of moles of oxygen needed to react with 32 moles of C4H10. Molar mass of CO2 44 gmol.
In one CO2 molecule there are two Oxygen atoms. CO2g 4 mol-3935 kJmol-1574 kJ. 2NH3 CO2 - CONH2 2 H2O balanced eq 2.
For this reaction the balanced chemical equation is 2H_2 O_2 - 2H_2O The mole ratios are determined using the coefficients of the substances in the balanced chemical equation. Zn2HCl-ZnCl2H2 25 mole Zn 6o mole HCl c. The molar ration of C O 2 and H 2 O produced by the combustion of one mole of hydrocarbon X is 5.
According to the equation below what is the mole ratio of O2 to H2O. 2NaHCO3 ----- Na2CO3 CO2 H2O. In the reaction we can see.
2 mol H_2. Moles of CO2 1244 0272 moles of CO2.

Question Video Deducing The Molar Ratio Of Reactants From A Balanced Reaction Equation Nagwa
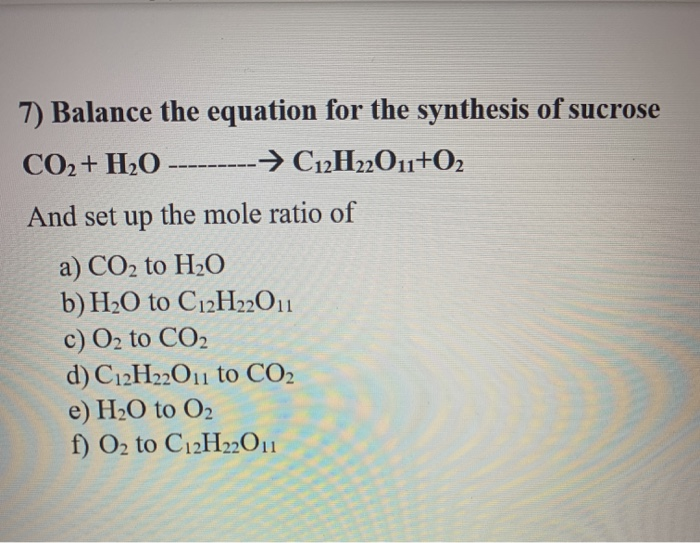
Solved 7 Balance The Equation For The Synthesis Of Sucrose Chegg Com
Chemistry Why Is Water H2o Heavier Than Carbon Dioxide Co2 Even Though The Latter Has 2 Atoms Of Oxygen Quora

No comments for "What Is the Mole Ratio of Co2 to H2o"
Post a Comment